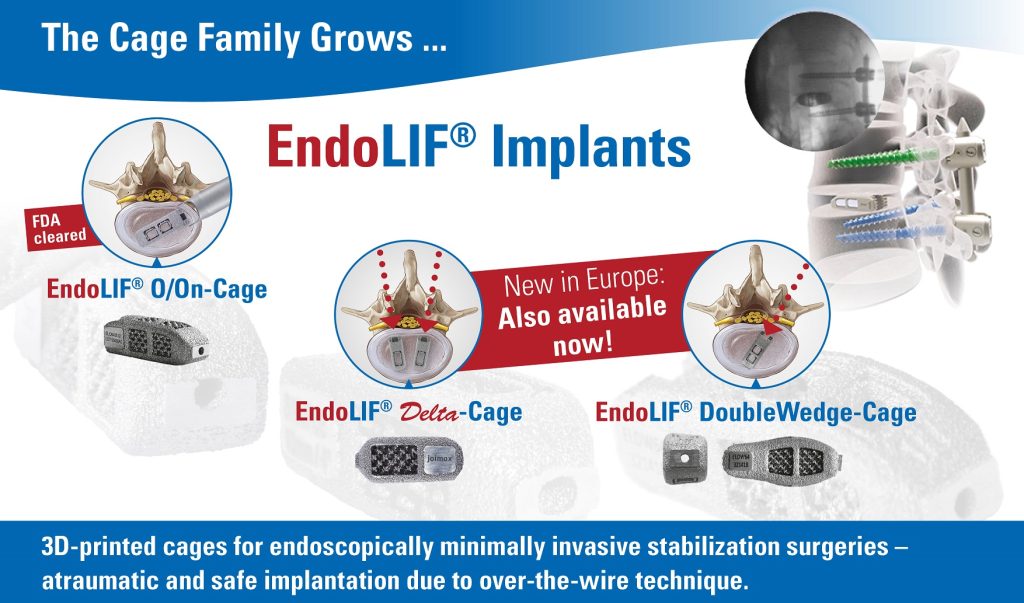
joimax® starts the official sale of its EndoLIF® Delta and DoubleWedge cages. All three members of EndoLIF® family CE approved.
Karlsruhe, GER– July 15, 2019 –joimax®, the Germany-based market leader of technologies and training methods for full-endoscopic minimally-invasive spinal surgery, announces the official sales launch of its complete EndoLIF® family. EndoLIF® is an instrument set for endoscopic-assisted minimally invasive lumbar interbody fusion. As already announced and showcased at earlier events, the EndoLIF® product line consists of three members now: the well-known EndoLIF® O/On-Cage, the EndoLIF® Delta Cage, and the latest EndoLIF® Double Wedge Cage. The complete EndoLIF® product family is now CE-approved and fully available. Moreover, the EndoLIF® O/On-Cage is FDA-approved.
All EndoLIF® Implants are manufactured using a 3D printing process, which ensures an open diamond cell structure. The cages are safely implanted via the guide wire and are fillable with bone or bone substitute material. With their rough and porous surface, the implants guarantee optimal bone ingrowth, stability and finally fusion.
The EndoLIF® Cage family provides the right implant for every patient and is the perfect solution for endoscopic spinal fusion. With the gentle and atraumatic access via gradual tissue dilatation, muscles remain intact leaving important biomechanical structures in place after surgery.
Like with any endoscopic procedure compared to open surgery, all advantages for fusion are now given with the EndoLIF® program also. With one EndoLIF® Instrument Set all three cages of the EndoLIF® family can be implemented.
Dr. Florian Zentz, (Munich, GER) emphasizes “the extraordinarily high primary stability of the EndoLIF® DoubleWedge-Cage”. He continues enthusiastically: “After insertion the cage sits very firmly in the intervertebral disc space and adjusts the angulation up to 18 degrees, almost directly enabling an optimal lordosis correction.”
Dr. Ralf Wagner, (Frankfurt, GER) is also convinced of the obvious advantages: “The Delta Cage is ideal for the posterior lumbar interbody fusion (PLIF) offering a gentle posterior alternative, especially at level L5-S1 with its large interlaminar window.
About joimax®
Founded in Karlsruhe, Germany in 2001, joimax® is the leading developer and marketer of complete systems for full-endoscopic and minimally invasive spinal surgery. With the Endoscopic Surgical Systms TESSYS® (transforaminal), iLESSYS® (interlaminar) and CESSYS® (cervical) for decompression procedures, MultiZYTE® for facet and sacroiliac joint pain treatment, EndoLIF® and Percusys® for minimally invasive endoscopically assisted stabilizations, established systems are provided, addressing a whole range of indications. All methods are supported by the latest generation electronical devices in the all new NAVENTO® navigated endoscopic tower. In procedures for herniated discs, stenosis, pain therapy or spinal stabilization treatment, surgeons utilize joimax® technologies to operate through small incisions under local or full anesthesia, via tissue and muscle-sparing corridors, and through natural openings in the spinal canal, e.g., the intervertebral foramen, the so-called "Kambin triangle".
Contacts
Press Contact Germany
joimax® GmbH
Antje Paulsen
antje.paulsen@joimax.com
+49-721-25514-214
Press Contact USA
joimax®, Inc.
Jose Elguera
jose.elguera@joimaxusa.com
+1 (949) 859 3472 301