IRVINE, CA – joimax®, the German-based market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery, is excited to announce the global launch of its new generation tower, NAVENTO® Navigation Endoscopic Tower. Optimized for endoscopic spine surgery with special settings for the safe treatment of sensitive structures, NAVENTO® will be featured at the North American Spine Society (NASS) meeting in Boston later this month and at EUROSPINE in Vienna this October.

NAVENTO®, the fully integrated fourth generation endo-tower, contains everything a surgeon needs to perform a successful procedure. The tower boasts updated design features for enhanced usability and is composed of six devices that work in unison to give the user the best possible surgical experience:
- Vitegra®, the brain of NAVENTO®, offers complete documentation and data management.
- Camsource® LED provides optimal clarity up to 4K resolution.
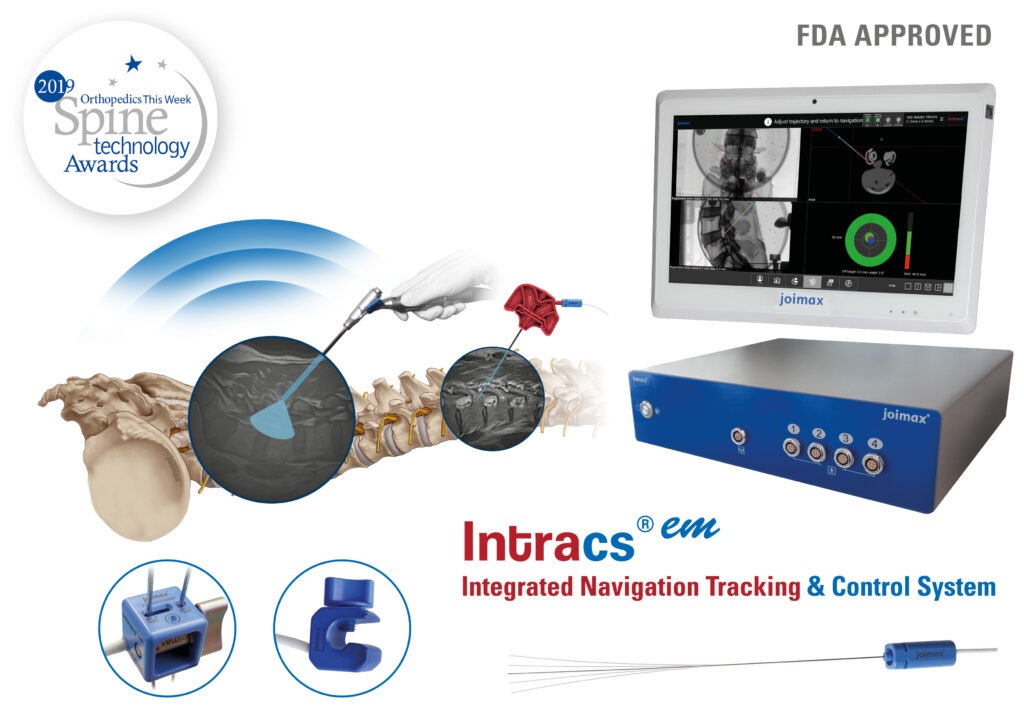
- Intracs®, the newest device from joimax®, delivers electromagnetic navigation at the instrument’s tip.
- Shrill®, the shaver drill system, with the newest generation recently launched, provides optimal RPMs and torque management.
- Endovapor® 2 poses electrosurgical offerings for monopolar and bipolar settings.
- Versicon®, delivers fluid and pressure regulation.
“joimax® continues to lead the endoscopic spine market through innovations in technologies aimed to increase the user experience and provide spinal patients with an ultra-minimally invasive treatment option,” stated Christoph Hofstetter, M.D., Ph.D., associate professor in the Department of Neurological Surgery at the University of Washington.
Wolfgang Ries, CEO and founder of the joimax® group, confirmed, “NAVENTO® pulls together the latest technologies forming one complete, seamlessly integrated spinal endoscopic package.”
NAVENTO®, along with the full suite of joimax® endoscopic technologies will be showcased at NASS, booth #3512, and at EUROSPINE, booth #04.