Karlsruhe, Germany – June 8, 2015 (BUSINESS WIRE) – The German company joimax®, developer of technologies and training methods for minimally invasive endoscopic spinal surgery, today announced it will introduce the company’s new iLESSYS® Delta system to the market at the 66th Annual Meeting of the German Society of Neurosurgery (DGNC) taking place in Karlsruhe, Germany from June 7-10.
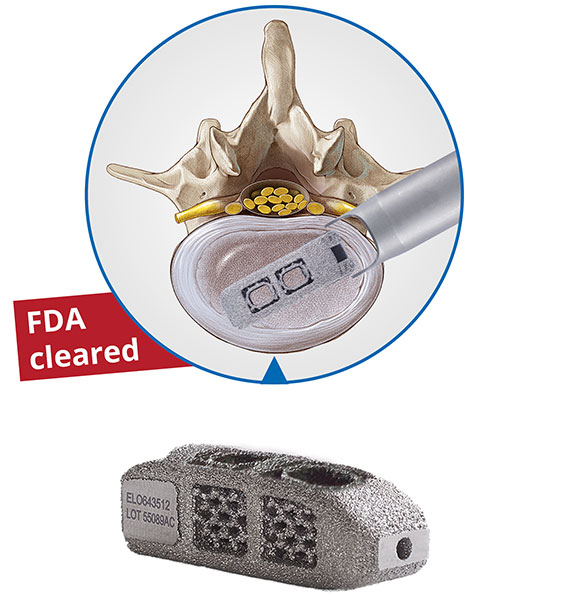
iLESSYS® Delta, an advanced development of the joimax iLESSYS® technology, is suitable for dorsal and dorso lateral treatment of central spinal canal stenosis. The iLESSYS® Delta system enables a large area to be decompressed by means of the interlaminar access, guided by endoscopic-assisted vision.
“Since the access to the spine is the same as with the already well-established iLESSYS® technique, usage is very straightforward.” said Dr. Guntram Krzok, orthopedic surgeon from Waltershausen, Germany after testing the new system at a joimax workshop early this year. “I was impressed by the new scope which delivers outstanding image quality. With the specialized instrument set, it is finally possible to achieve decompression of central spinal canal stenosis.”
The iLESSYS® Delta instrument set includes specially developed tools that enable extensive, yet gentle, tissue-sparing decompression. The 6 mm working channel of the new endoscope allows for the use of large shaver blades designed for bone resection and endo-kerrisons. An additional option is the plastic (PPSU) working tube for unimpaired X-ray examination.
“iLESSYS® Delta is a logical addition to our portfolio. Not only is the system suitable for endoscopic decompression, but it also allows the parallel implantation of cages. This way we can achieve dorsal decompression and stabilization in one step,” comments Wolfgang Ries, CEO and founder of joimax.
About joimax
Founded in Karlsruhe, Germany, in 2001, joimax® is one of the leading medical device companies in minimally invasive spinal surgery („joined minimal access“). The company’s U.S. subsidiary was established in Irvine, California, in 2005. The company is primarily focused on the development, production and marketing of technologies and methods for minimally invasive endoscopic spinal surgery. joimax is active in 40 countries around the globe and its methods have been successfully employed in approximately 130,000 surgeries. With a special focus on education, the company provides surgeons with specialized technique training through the three-step joimax CM3 education program. This program includes visitations, cadaver workshops and live-surgery support.
 both the surgeon and their patients in the U.S.,” said Wolfgang Ries, Founder and CEO of joimax®. This announcement is in alignment with the reimbursement decision of NICE, UK, in April of 2016, to fully reimburse endoscopic procedures.
both the surgeon and their patients in the U.S.,” said Wolfgang Ries, Founder and CEO of joimax®. This announcement is in alignment with the reimbursement decision of NICE, UK, in April of 2016, to fully reimburse endoscopic procedures.