Karlsruhe (GER) / Irvine, CA – December 3, 2019 – joimax®, the Germany-based market leader of technologies and training methods for full-endoscopic minimally-invasive spinal surgery, celebrated resounding success at this year’s German Spine Society (DWG), held November 28 to 30 in Munich, Germany. joimax® presented its newest product innovations and hosted several informative sessions to rave reviews, exceeding expectations.
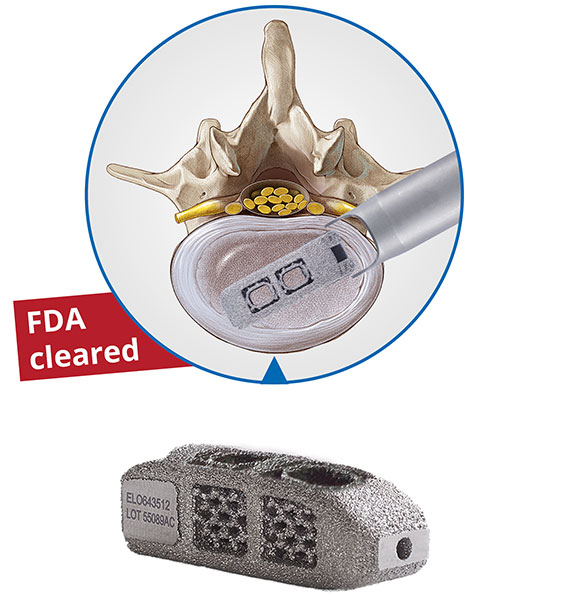
Among the products showcased was a new member of the joimax® TESSYS® family, TESSYS® Thx, a system specifically developed for the symptomatic thoracic deherniation and decompression treatment. Surgeries utilizing TESSYS® Thx can avoid complications often associated with traditional open and microsurgical approaches, such as significant muscle damage, bony resection, and morbidities.

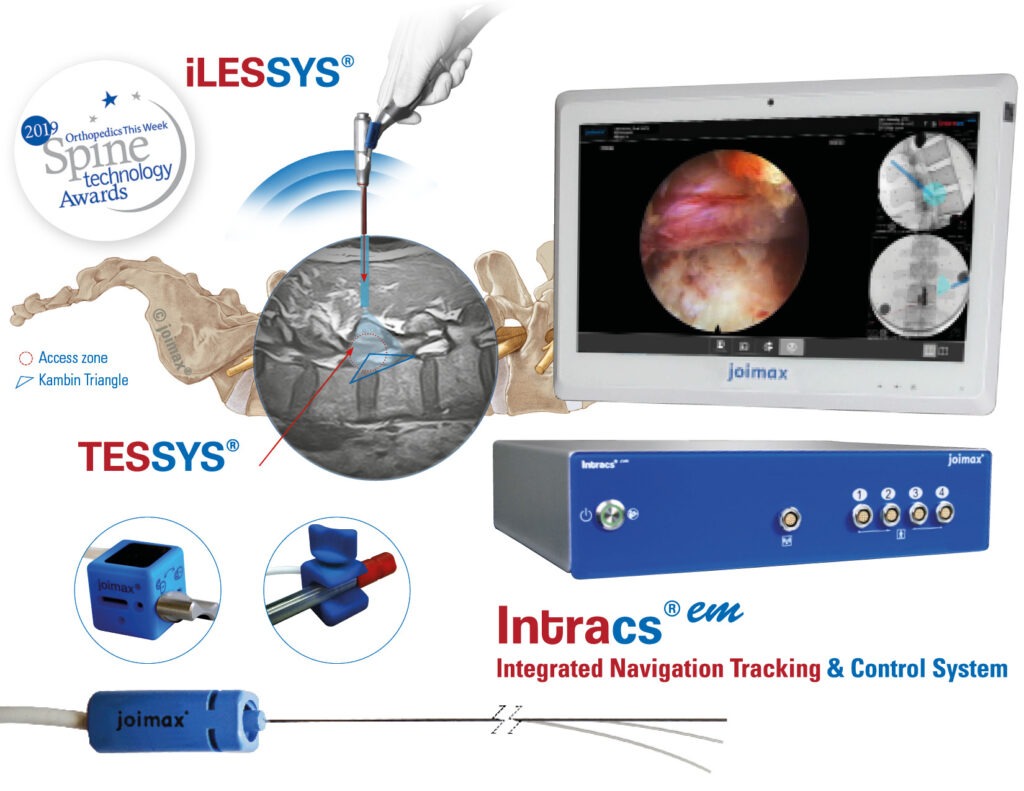
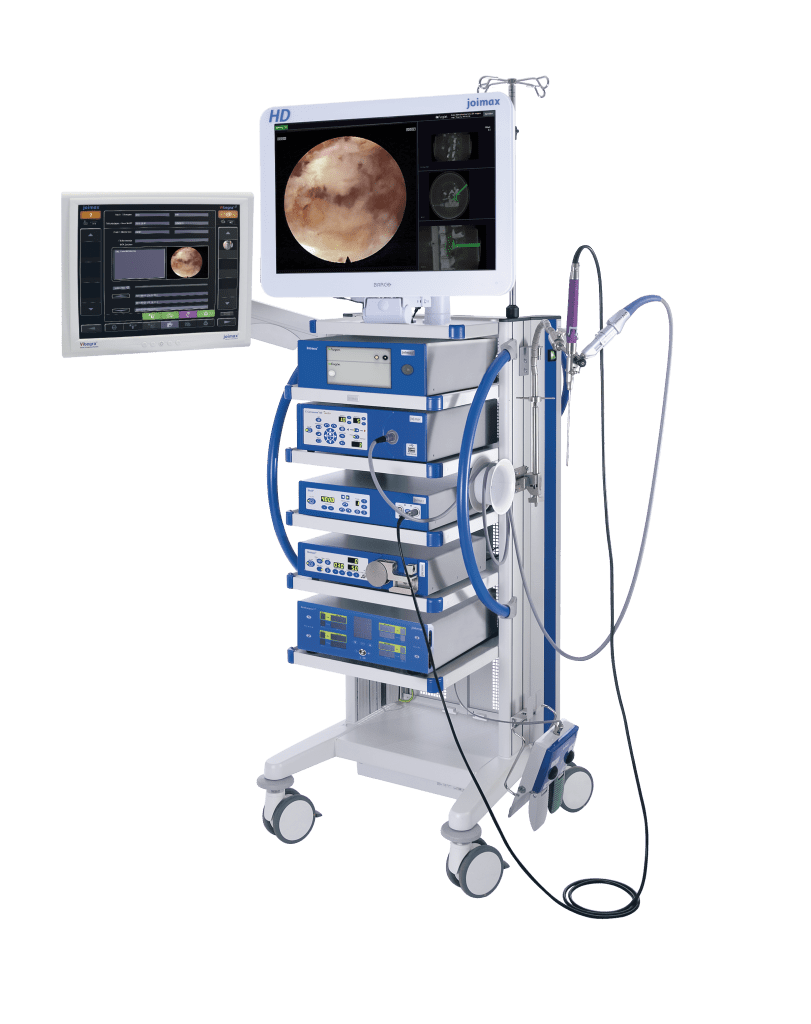
joimax® also presented Intracs®em, the first electromagnetic system for endoscopic spine applications that can navigate instruments directly at the tip, if required. This navigation system combines side and AP images of the C-arm with the CT axial scan, providing surgeons with meaningful views of the target region. Intracs® em received its CE mark earlier this year and has been successful throughout Europe and Asia. FDA approval is pending; expected by year’s end.
Moreover, joimax® presented its Endoscopic Spine Academy ESPINEA®. This modular training program, divided into a Pre-Clinical Training with anatomical models, endoscopic tools, and simulators, and a Clinical Training, part of the joimax® CM3 program consisting of visitation, hands-on workshops, and the first surgery, offers surgeons specific support to optimize and significantly shorten the individual learning curve.
Additional highlights included, the daily Meet the Expert Sessions, a Needle Placement Challenge with attractive prizes, and a lunch symposium on “Endoscopy in the Course of Time”.
joimax® CEO and Founder Wolfgang Ries states, “Our unmatched attention to the needs of surgeons enables us to be one step ahead. The reception of our products at this convention reinforced our commitment to endoscopy and underscored the importance of showcasing innovative products such as Intracs®em. By participating in national and international spine congresses, we are able to achieve broad market penetration for the benefit of the patient”.