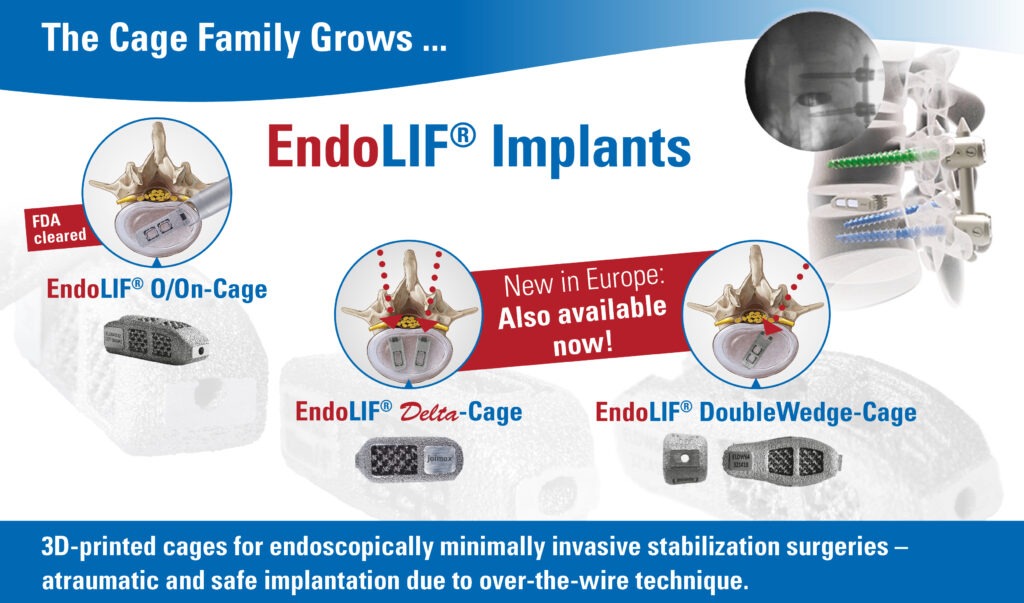
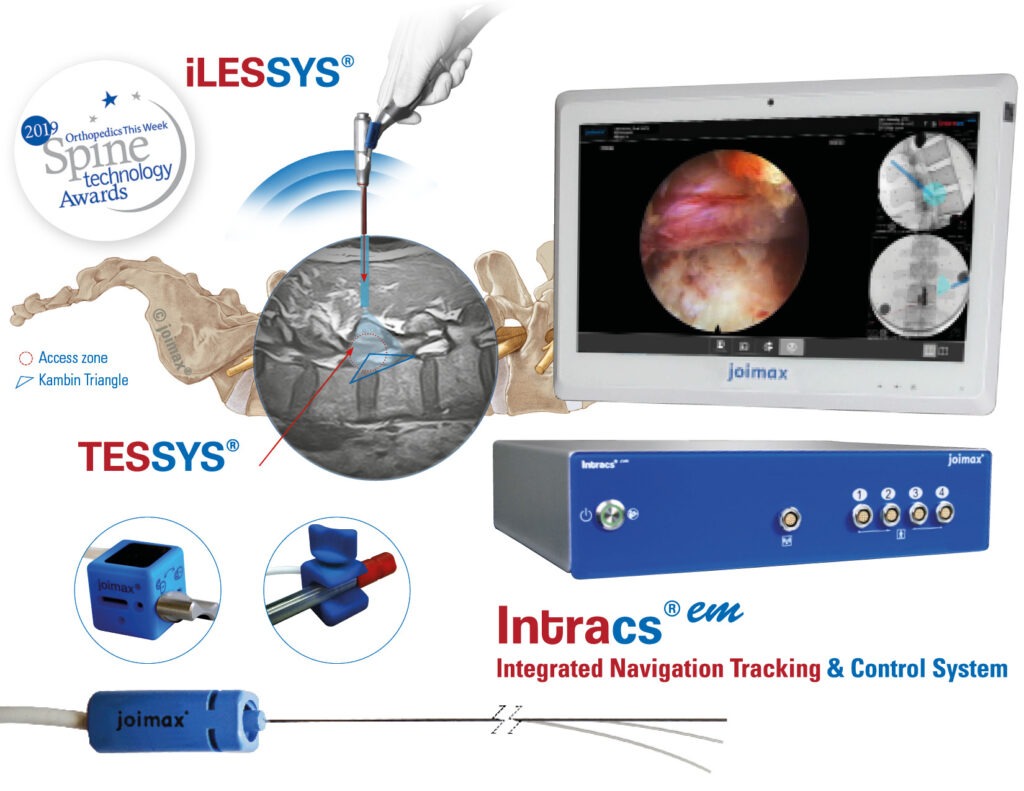
SAVANNAH, GA – joimax®, a leading developer and marketer of complete systems for endoscopic minimally invasive spinal surgery, and Red One Medical, a Service-Disabled Veteran-Owned Small Business, announced their partnership today. The partnership enables both companies to continue their missions to improve patient care and surgical outcomes with leading edge medical technology and devices.
Red One Medical Founder and CEO Charles Pollak shared, “I am excited to join forces with joimax® to offer their innovative technologies, systems and methods for endoscopic minimally invasive spinal surgery to VA and DoD facilities across the nation, further supporting the government’s goal to improve outcomes and improve efficiency in these facilities.”
Matt Cronin, senior VP of sales and marketing for joimax®, is a U.S. Navy veteran and hailed this partnership: “This is a pivotal moment for joimax® and our long history. We are honored to be a part of improving health care for our many veterans and our brave U.S. military personnel, as well as for their dependents. Our working together with Red One Medical will make endoscopic spine surgery more accessible to this patient population, where a quicker return to both duty and a more active life, is so important.”
About Red One Medical:
Red One Medical is a CVE-certified Service-Disabled Veteran-Owned Small Business (SDVOSB) located in Savannah, GA. Wholly owned and operated by a combat veteran, Red One Medical is a leading medical device distributor and wholesaler serving the Department of Veterans Affairs (VA) medical centers and Department of Defense (DoD) hospitals. Red One Medical donates a portion of profits to leading charities as part of its mission to support veterans, military families and their communities. To learn more, visit http://www.redonemedical.com/