Kyverment® - PMMA Bone Cement
Kyverment® is a radiopaque PMMA cement especially developed for the treatment of vertebral body fractures. It is optimally combined with the SPASYTM kyphoplasty system.
PMMA Bone Cement for Kyphoplasty and Vertebroplasty
The composition of Kyverment® has proven itself on the market for many years.
With its high content of microdispersed and non-sedimentable barium sulfate the injection can be well controlled and immediately visualized under fluoroscopic control. Kyverment® has an outstanding viscosity and excellent compression characteristics.
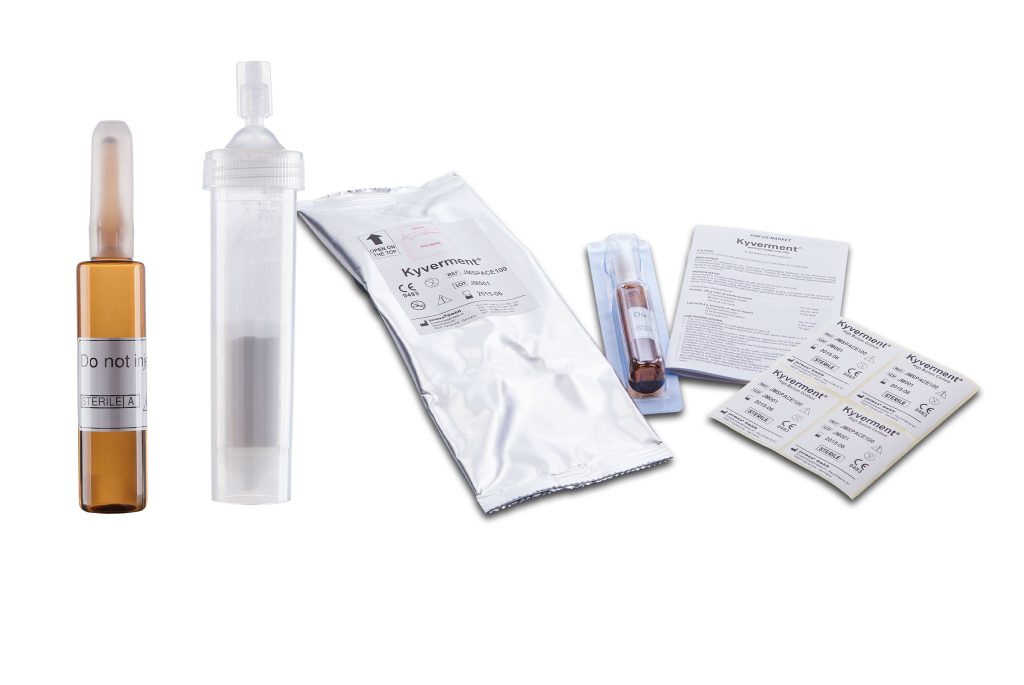

Features of Kyverment®
- Easy preparation in practical mixing cylinder
- Optimal fluidity for easy and fast application
- Outstanding viscosity for excellent control during the injection
- Immediate visualization of the injected cement, thanks to high content of barium sulfate USP microdispersed and non-sedimentable
- Adequate volume (22 ml) for one or more injections
- Excellent compression characteristics
Disclaimers
Certain products may not be approved for sale in all countries.
Bibliography
- Mpotsaris A, Abdolvahabi R, Hoffleith B, Nickel J, Harati A, Loehr C et al. Percutaneous vertebroplasty in vertebral compression fractures of benign or malignant origin: a prospective study of 1188 patients with follow-up of 12 months. Dtsch Arztebl Int 2011; 108: 331–338.
- Health Quality Ontario. “Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: A Systematic Review.” Ontario Health Technology Assessment Series 16, no. 11 (2016): 1–202.
- Khan, Majid, and Sergiy V. Kushchayev. “Percutaneous Vertebral Body Augmentations: The State of Art.” Neuroimaging Clinics of North America 29, no. 4 (November 2019): 495–513. https://doi.org/10.1016/j.nic.2019.07.002.
- Onggo, James Randolph, Julian T. Maingard, Mithun Nambiar, Aaron Buckland, Ronil V. Chandra, and Joshua A. Hirsch. “Role of Vertebroplasty and Balloon Kyphoplasty in Pathological Fracture in Myeloma: A Narrative Review.” European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 30, no. 10 (October 2021): 2825–38. https://doi.org/10.1007/s00586-021-06955-5.
Scientific Background
[1] Endoscopic Lumbar Interbody Fusion and Minimally Invasive Transforaminal Lumbar
Interbody Fusion for the Treatment of Lumbar Degenerative Diseases: A Systematic Review and Meta-Analysis
Kou Y, Chang J, Guan X, Chang Q, Feng H. World Neurosurg. 2021 Aug;152:e352-e368
[2] Endoscopic Techniques for Lumbar Interbody Fusion: Principles and Context
Zheng B, Shaaya E, Feler J, Leary OP, Hagan MJ, Bajaj A, Fridley JS, Hassel F, Gardocki R, Grau CR, Lewandrowski K-U, Telfeian AE; BioMed Research International (2022): e4979231
[3] Comparison of Clinical Outcomes and Complications Between Endoscopic and Minimally Invasive Transforaminal Lumbar Interbody Fusion for Lumbar Degenerative Diseases: A Systematic Review and Meta-analysis
Guo H, Song Y, Weng R, Tian H, Yuan J, Li Y; Global Spine J 2022


