KARLSRUHE, Germany – (BUSINESS WIRE) – German-based joimax®, the market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery, is excited to announce the full registration of all its products for India, one of the fastest growing endoscopic spine surgery markets worldwide.
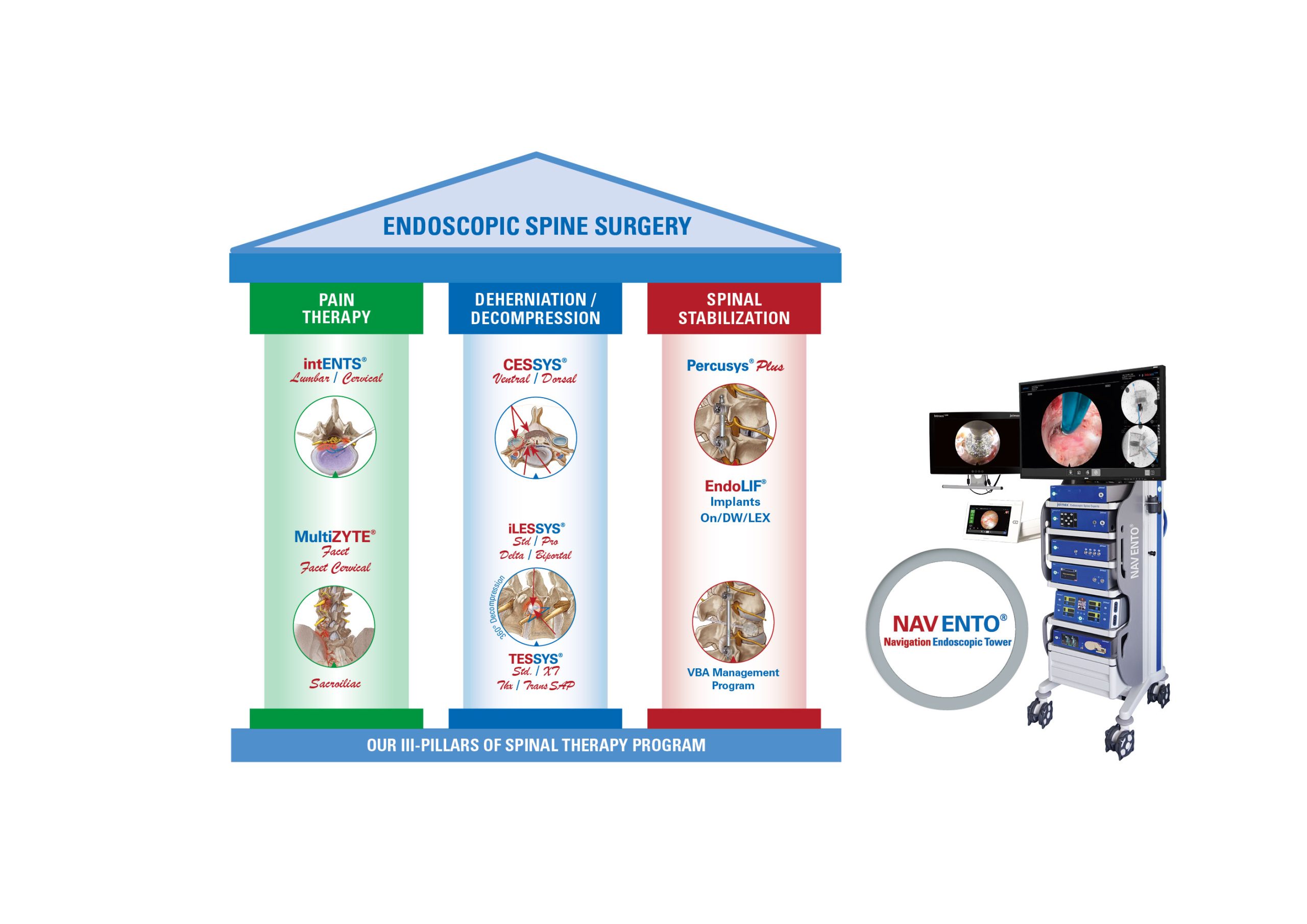
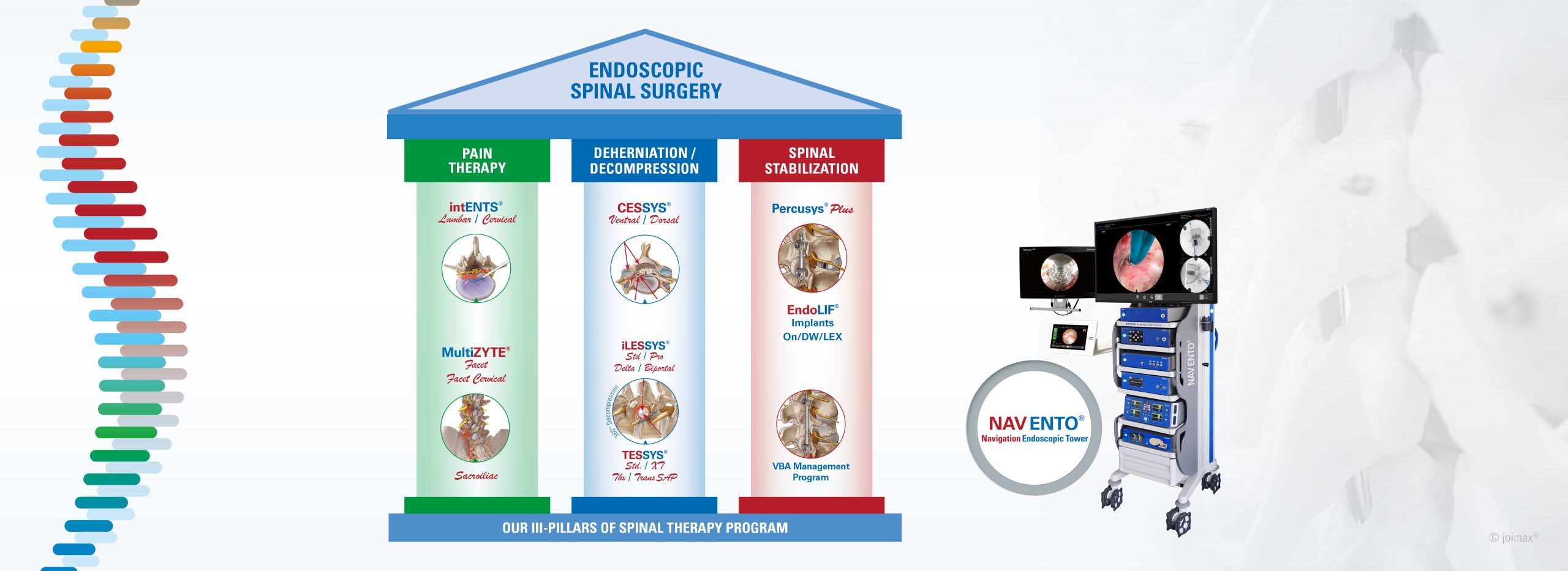
The Indian registration includes all joimax® Class II and III, as well as Class I instruments, which were approved in 2023. Now, the Indian health community has access to all the latest spinal endoscopic technologies invented, developed and manufactured by joimax®.
“India is the biggest country in the world; it’s ready for emerging technology businesses like endoscopic spinal surgery,” states Santhosh Mechery, Director Spectramed Healthcare (P) Ltd. “We can’t wait to roll-out the complete joimax® portfolio in our country and enable so many patients to profit from this innovative, minimally invasive endoscopic spine surgery.”
“Earlier this year, joimax® received its CE MDR approvals, granted by TÜV-South — an important step for marketing our existing products under the new European regulation, which opened a backlog of products to India,” states joimax® Founder and CEO Wolfgang Ries. “We’re already active in more than 60 countries! These partners are eager to launch the newest joimax® treatment technologies.”
In Fall 2022, joimax®, in cooperation with Spectramed Healthcare, launched its education and training program, Endoscopic Spine Academy (ESPINEA®), in India, which has since been rolled out worldwide. ESPINEA® was founded in 2019, accredited by the Royal College of Surgeons of Edinburgh in 2020, and this year, is celebrating its 5th anniversary. The program allows for the highest quality training of joimax® products in any market, serving as a hands-on learning facility for endoscopic spinal surgery.