About Us
joimax® designs and delivers advanced devices and systems for spine surgery worldwide.
Executive Leadership Team
Our senior management team brings together innovative thinking, strategic execution, and decades of industry expertise to drive joimax® forward.

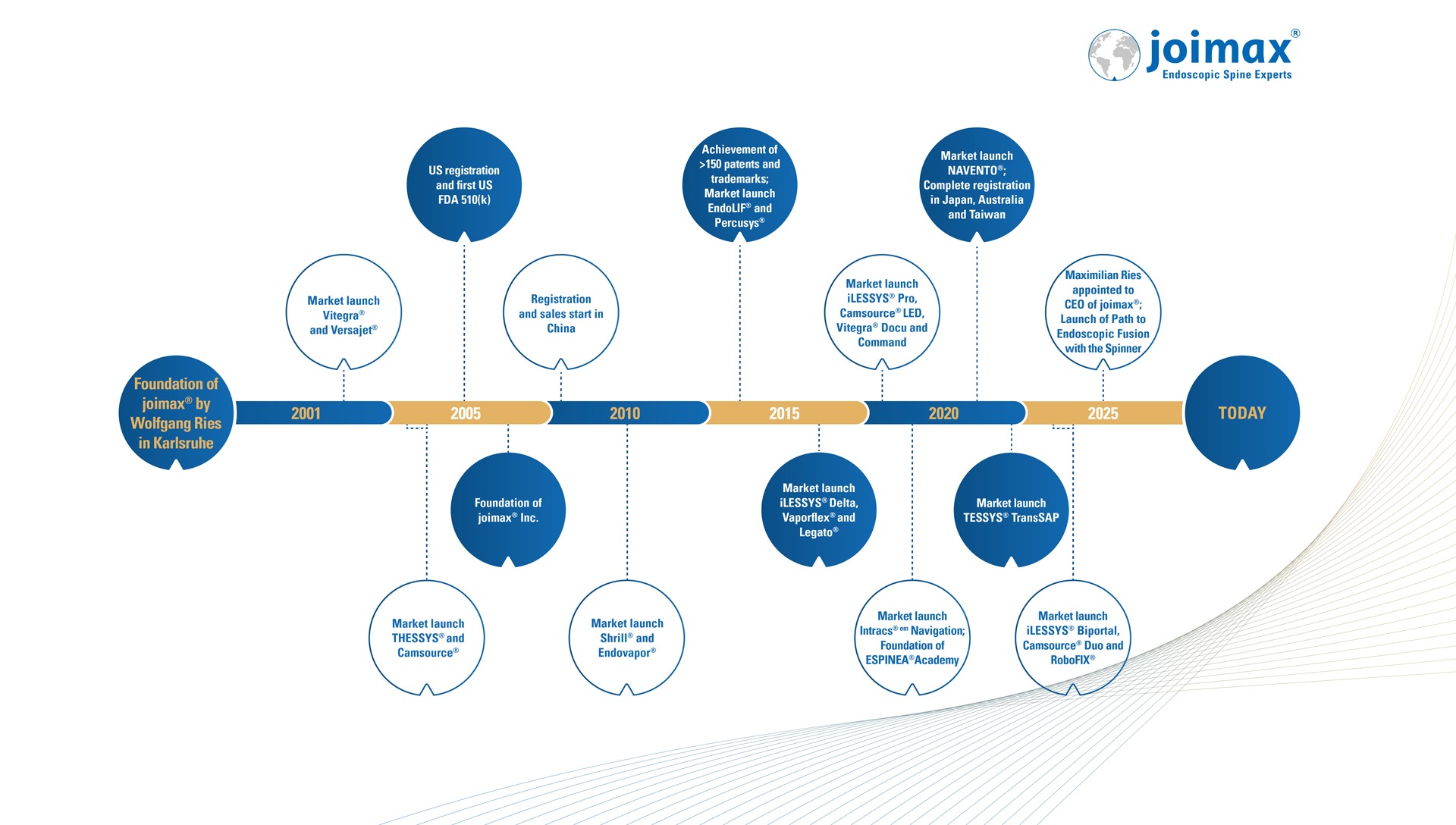
Maximilian Ries, CEO at joimax®, has been part of the family business since 2009. As the son of founder Wolfgang Ries, Maximilian has played a crucial role in the company’s growth. He initially served as Project Manager, then Product Manager, followed by Integration & Operations Manager at joimax® GmbH, before becoming Managing Director of joimax® Inc. in the USA in 2016. In 2023, he was appointed Executive Vice President (EVP), overseeing global operations from the joimax headquarters in Germany. In June 2025, he was appointed CEO and has since been leading the joimax® Group. Maximilian holds a degree in construction engineering and business administration from VWA in Karlsruhe. His expertise meets joimax® needs to adapt quickly to new challenges and continued driving its expansion.

Rainer Schmitz, CFO/COO of joimax®, brings over 40 years of professional experience in finance and controlling. With nearly two decades at joimax®, Rainer has been an integral part of the company since joining in October 2006. Prior to joimax®, he held key commercial management roles, including General Manager of Finance/Controlling at TerraTec Electronic GmbH, a global leader in the consumer electronics sector. His expertise includes strategic corporate planning, process optimization, finance, controlling, and human resources. Rainer holds a degree in Business Administration and completed a trainee program in South Africa, along with further studies at the University of Hagen in Germany.

With over 43 years of professional experience, including 37 years in medical technology and 6 years in telecommunications, Wolfgang Ries, founder and Executive Chairman of joimax®, brings deep expertise and leadership to the company. Before founding joimax® in 2001, he held key roles in sales, product, and marketing management. Notably, he served as VP of Business Development Europe at Baxter CardioVascular Group (now Edwards LifeScience) and Managing Director at Saphir Medical. Wolfgang studied medicine for several semesters at the University of Vienna and has completed training in communications technology as well as various management programs, including the MBA Executive course “Leadership 2000” at IMD Lausanne.

Tony Troncale, President of joimax® Inc., is a senior business executive with more than 30 years of experience in the medical technology industry and clinical practice. As a proven commercial leader, Tony has contributed to the development of start-ups and Fortune 500 companies in the fields of orthopedics, neurology, and spine. Tony joined joimax® Inc. in 2021 and was promoted to General Manager in 2023 and President in 2025. Since his start at joimax®, Tony has not only expanded his responsibilities but has also leveraged the growing interest in endoscopic techniques to successfully further expand the company’s sales and marketing activities in the USA. He holds an MBA from UNC, an MHS from Duke University, and a BS in Physiology from UC San Diego.
Commercial Leadership Team

Chris Leever
VP of Business Operations & Finance

Katherine Ray, MS, MBA
Director of Global Marketing

Clemens Barthold
Director of Product Management

Joe O’Connor
Vice President of Sales, US

Johan De Ranter
Senior Director of Sales & Marketing, EMEIA

Dominik Beck
Director of Sales, DACH & FRANCE

Carrie Khoo
Senior Director of Sales, APAC

Laura Rigoni
Senior Director of Sales, LATAM

Jarred Shewey
Director of Clinical Education

Levon Zanadvorov, MD
Medical Director