Irvine, CA and Karlsruhe, Germany – October 25, 2016 – joimax®, the global acting German developer and marketer of technologies and training methods for endoscopic minimally-invasive spinal surgery, will again exhibit at the annual meeting of the North American Spine Society (NASS) 2016, taking place from October 26 – 29 in Boston, USA. Prior to the convention, on Monday, October 24, joimax® will hold a User Meeting followed by a workshop at the Harvard Medical School Conference Center. On Tuesday, October 25, the NASS CME-Lab with a joimax® station will take place. During the exhibition a group of internationally respected spine experts will also give presentations in a forum at the joimax® booth.
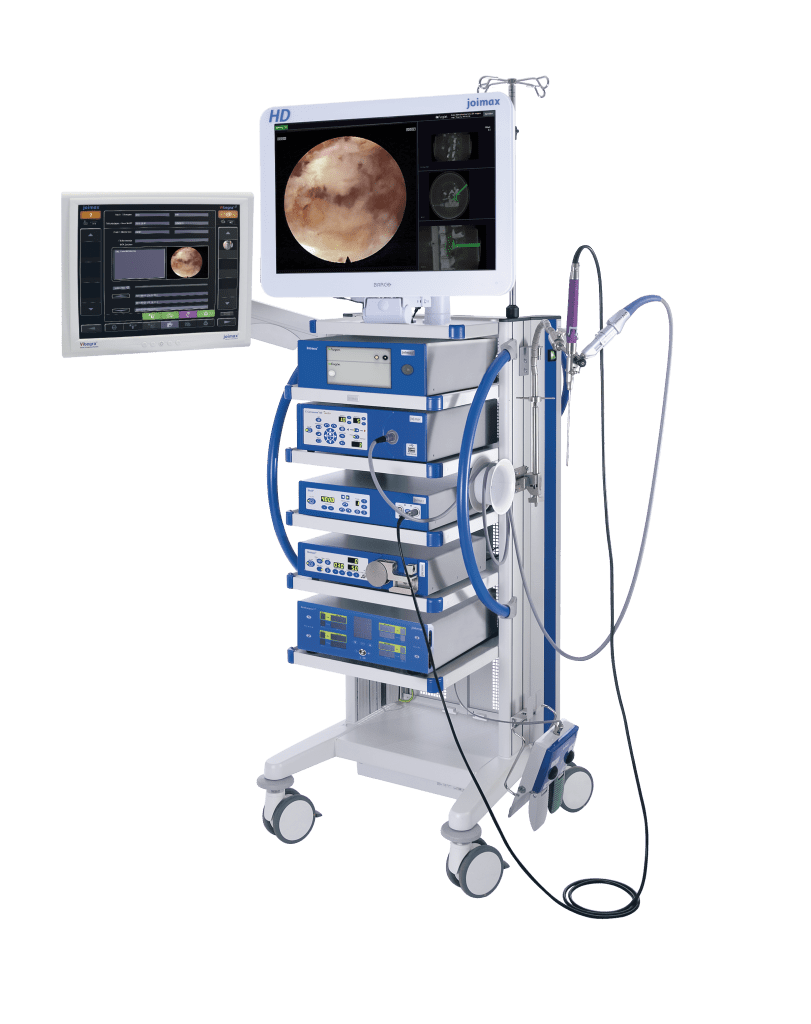
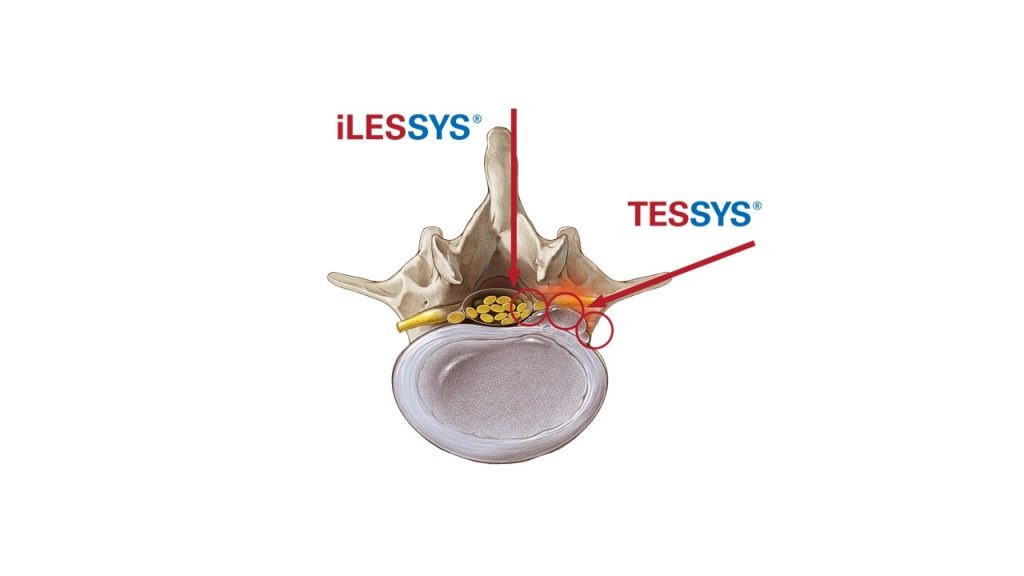
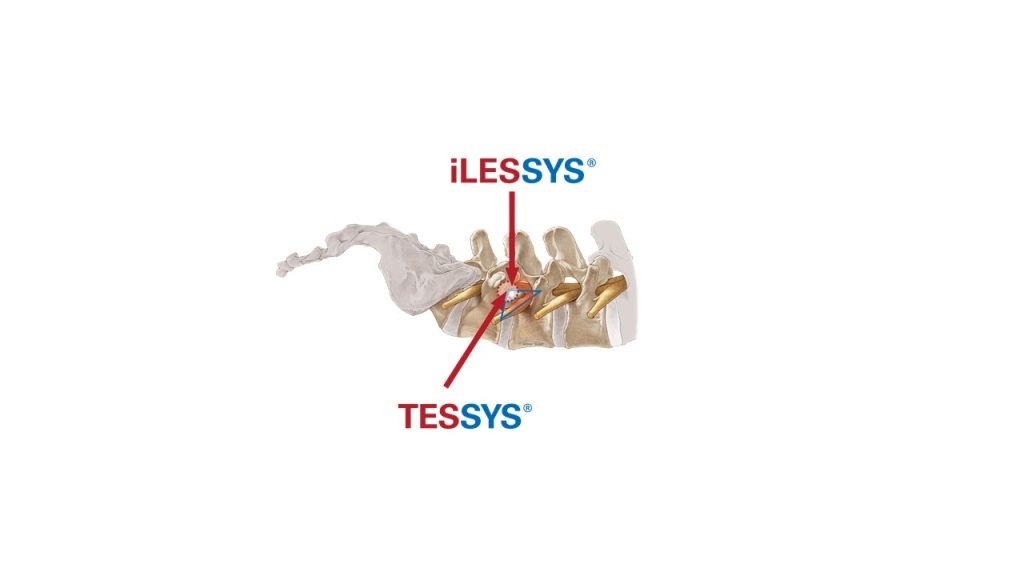
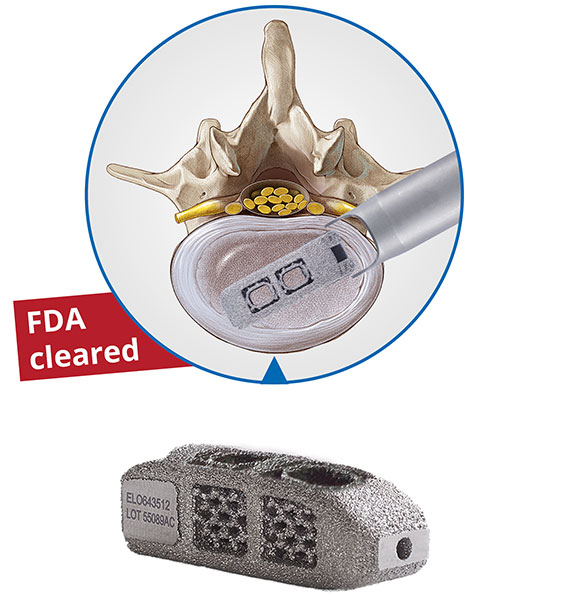
Key topics of the joimax® User Meeting will be centered around the endoscopic treatment of degenerative spinal diseases on the basis of the joimax® portfolio products with TESSYS®, CESSYS®, iLESSYS® Delta systems as well as EndoLIF® and Percusys®. A group of spine experts will share their expertise and extensive knowledge on the treatment of spinal diseases. The meeting will be completed with a cadaver lab with 3-4 stations at the Conference Center of the Harvard Medical School to train attendees on the whole range of endoscopic minimally-invasive spine surgery applications based on the joimax® techniques.
Tuesday’s NASS CME-Lab will be chaired by Professor Michael Y. Wang, M.D. FACS, University of Miami Miller School of Medicine, Miami, Florida, another pioneering spine surgeon and joimax® faculty member, who recently published a special edition in the Journal of Neurosurgery called Neurosurgical Focus, focused on Endoscopic Spine Surgery.
Additionally, joimax® will host a “Meet the Expert Forum” with presentations at its booth given by Dr. Ralf Wagner and Dr. Erik Traupe, both from Germany, and also Dr. Menno Iprenburg from the Netherlands. Topics discussed will include the benefits of spinal endoscopic transforminal and interlaminar decompression methods over traditional ones. joimaxs’® novel treatment options for advanced indications enjoy a fast growing adoption rate of its endoscopic technologies worldwide will also be discussed.

“In the context of the annual NASS meetings’ we aim to share the latest information, innovative techniques and best practices with renowned spine care professionals from around the world. These joimax® activities will satisfy the requirements of spine specialists to take advantage of the latest innovations and technologies in endoscopic minimally-invasive spine surgery,” says Wolfgang Ries, CEO and founder of joimax®.
“With our existing product range as well as with our newly launched products – the MultiZYTE® SI endoscopic sacroiliac joint therapy set, MultiZYTE® RT for endoscopic minimally-invasive treatment of the facet joint and the new Intracs® Intraoperative Navigation Tracking & Control System, joimax® uniquely enhances its endoscopic minimally-invasive product portfolio to fully meet users’ needs,” he continues.