Karlsruhe (GER) – joimax®, the German-based market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery, is pleased to announce a new partnership with Transmedic Group, a top medical device distributor headquartered in Singapore. In this exclusive deal, Transmedic will represent joimax® in Singapore, Hong Kong, Philippines and Indonesia.
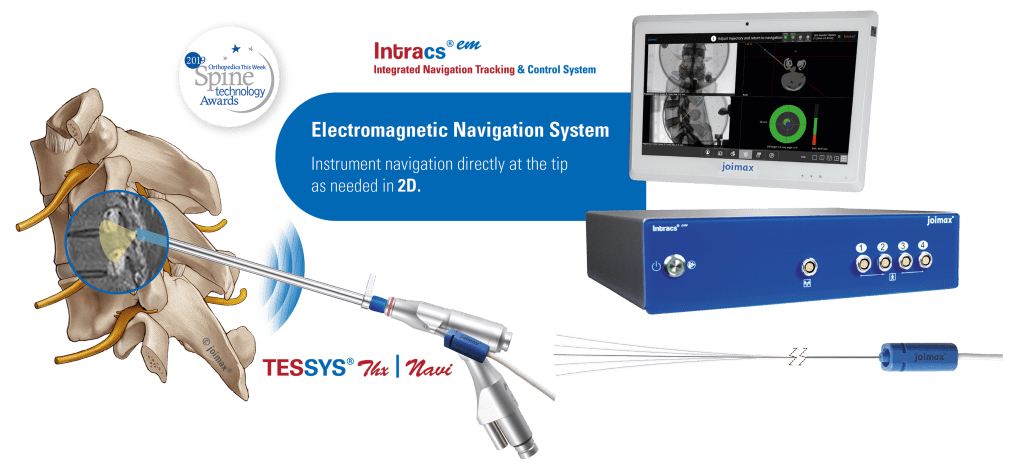
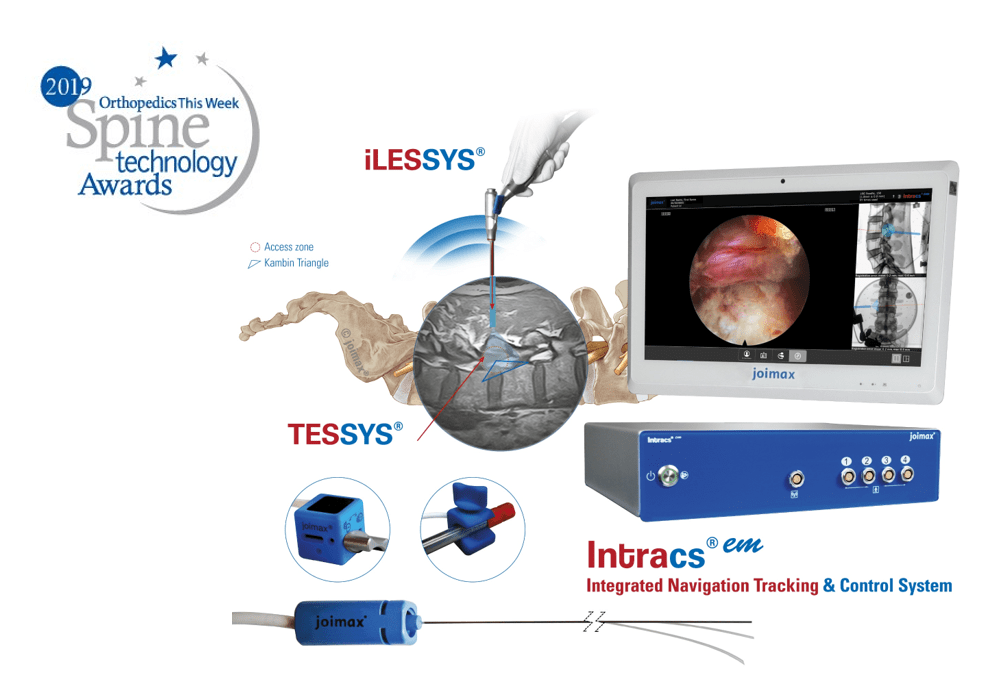
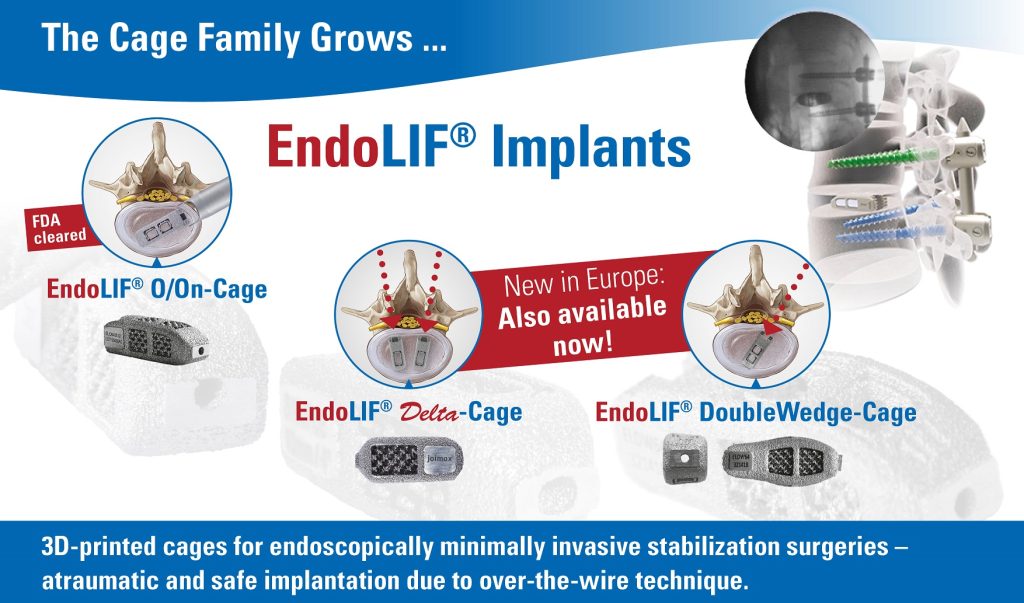
The long-term partnership will provide a broader surgical choice for patients considering endoscopic minimally invasive spinal surgery in these markets. With recent advances in the field of endoscopic discectomy (ED), the procedure has potential to supersedemicrodiscectomy (MD) as the gold standard of care in management of lumbar disc disease, as the latest literature suggests.
“joimax® is very much looking forward to working with Transmedic Group,” says joimax® Founder and CEO Wolfgang Ries. “With the rising popularity of endoscopic approaches in the management of spinal diseases, we’re able to deliver the highest standard of patient care, strengthen our sales activities, and enter into another important partnership across APAC.”
Teo Kee Meng, managing director of Transmedic Group commented, “We’re very pleased to partner with joimax®. With their technological advancements and use of new spinal access corridors, a major prevention of spinal structure collateral damage is assured, allowing for significantly reduced complications and improved postoperative recovery times.”

About Transmedic Group
Transmedic Group was founded in 1980 with the aim of becoming the top specialist in the advanced medical technology area in Southeast Asia. Headquartered in Singapore, the privately held company has since grown its presence in seven countries with more than 500 employees, and is well-renowned as the leading provider of latest technologies in healthcare.