EndoLIF® - Endoscopic Lumbar Interbody Fusion
Spinal stabilization represents an essential treatment option for a number of spine conditions. With our minimally invasive procedures and associated implants,
we offer a safe and effective alternative to conventional surgical stabilization techniques. The joimax® portfolio covers everything from endoscopically-assisted implantation of intervertebral cages to percutaneous screw-rod systems and products for vertebral body augmentation, including a bone cement.
The EndoLIF® Concept
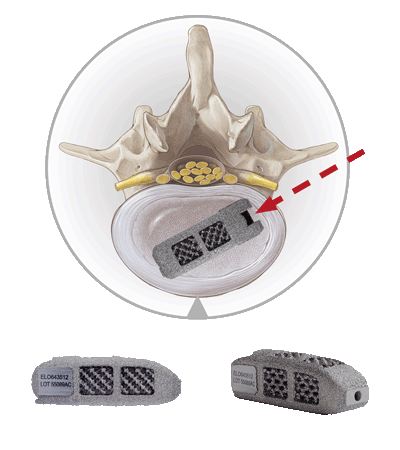
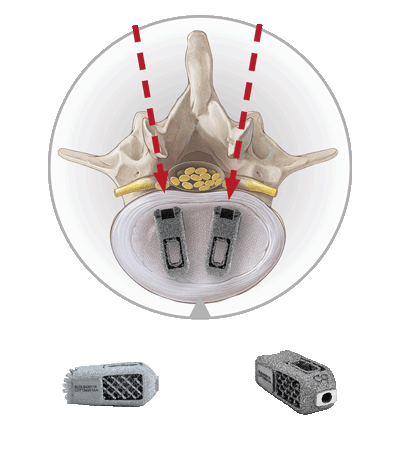
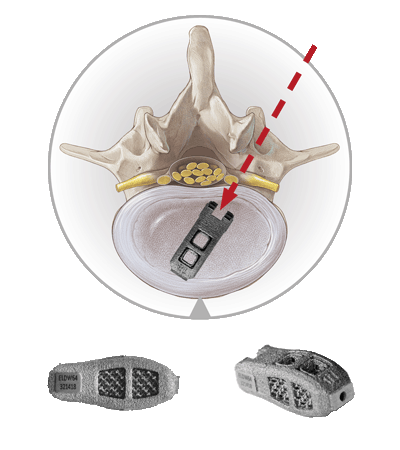
The EndoLIF® access is particularly tissue-conserving and allows for endoscopically-assisted implantation of intervertebral cages.
It works exceptionally well with our EndoLIF® cage portfolio, comprising additively manufactured titanium alloy implants. Just like with our other techniques the procedure is safely guided over a wire.

The EndoLIF® method is derived from the proven TESSYS® access, however experience with this system is not mandatory.
EndoLIF® offers the possibility of tissue conservation and gentle preparation of the vertebral disc space with endoscopic assistance. There is no need to remove stabilizing dorsal bone structures. Therefore, a fusion can be achieved without permanent structural damage to the bone or the multifidus muscle, which is vital for spinal stability.
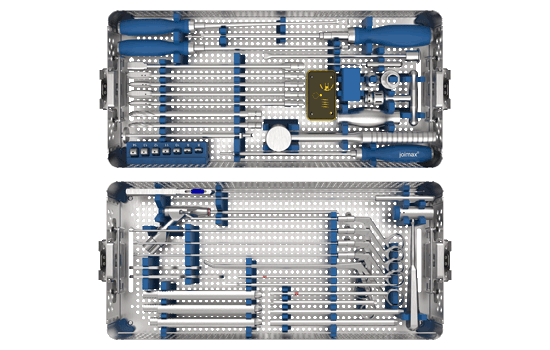
The clearly structured EndoLIF® instrument set consists of two trays: one tray for the endoscopically assisted access and one tray for the preparation of the intervertebral disc space and implantation of the EndoLIF® cages.
The EndoLIF® method is derived from the proven TESSYS® access, however experience with this system is not mandatory.
EndoLIF® offers the possibility of tissue conservation and gentle preparation of the vertebral disc space with endoscopic assistance. There is no need to remove stabilizing dorsal bone structures. Therefore, a fusion can be achieved without permanent structural damage to the bone or the multifidus muscle, which is vital for spinal stability.

The clearly structured EndoLIF® instrument set consists of two trays: one tray for the endoscopically assisted access and one tray for the preparation of the intervertebral disc space and implantation of the EndoLIF® cages.
Selected Indications
- Degenerative disc disease
- Mechanical instability
- Spondylolisthesis grade I or II (Meyerding)
- Secondary instabilities following surgical procedures on the lumbar spine

Innovative Solutions » Unique Benefits
EndoLIF® cages are designed for both minimally invasive endoscopic and open (PLIF/TLIF) fusion surgery of the lumbar spine to provide surgeons with an optimal treatment method for spinal stabilization. They consist of a titanium alloy (Ti6Al4V) and have exceptionally high surface roughness, which is favorable for this application.
The EndoLIF® cages have a characteristic diamond cell structure that can be filled with autologous bone or bone substitute to facilitate bone ingrowth. All cages are canulated and can be implanted safely using the “Over-the-Wire Technique”.

Disclaimers
Certain products may not be approved for sale in all countries.
Scientific Literature for EndoLIF®
[1] Endoscopic Lumbar Interbody Fusion and Minimally Invasive Transforaminal Lumbar
Interbody Fusion for the Treatment of Lumbar Degenerative Diseases: A Systematic Review and Meta-Analysis
Kou Y, Chang J, Guan X, Chang Q, Feng H. World Neurosurg. 2021 Aug;152:e352-e368
[2] Endoscopic Techniques for Lumbar Interbody Fusion: Principles and Context
Zheng B, Shaaya E, Feler J, Leary OP, Hagan MJ, Bajaj A, Fridley JS, Hassel F, Gardocki R, Grau CR, Lewandrowski K-U, Telfeian AE; BioMed Research International (2022): e4979231
[3] Comparison of Clinical Outcomes and Complications Between Endoscopic and Minimally Invasive Transforaminal Lumbar Interbody Fusion for Lumbar Degenerative Diseases: A Systematic Review and Meta-analysis
Guo H, Song Y, Weng R, Tian H, Yuan J, Li Y; Global Spine J 2022