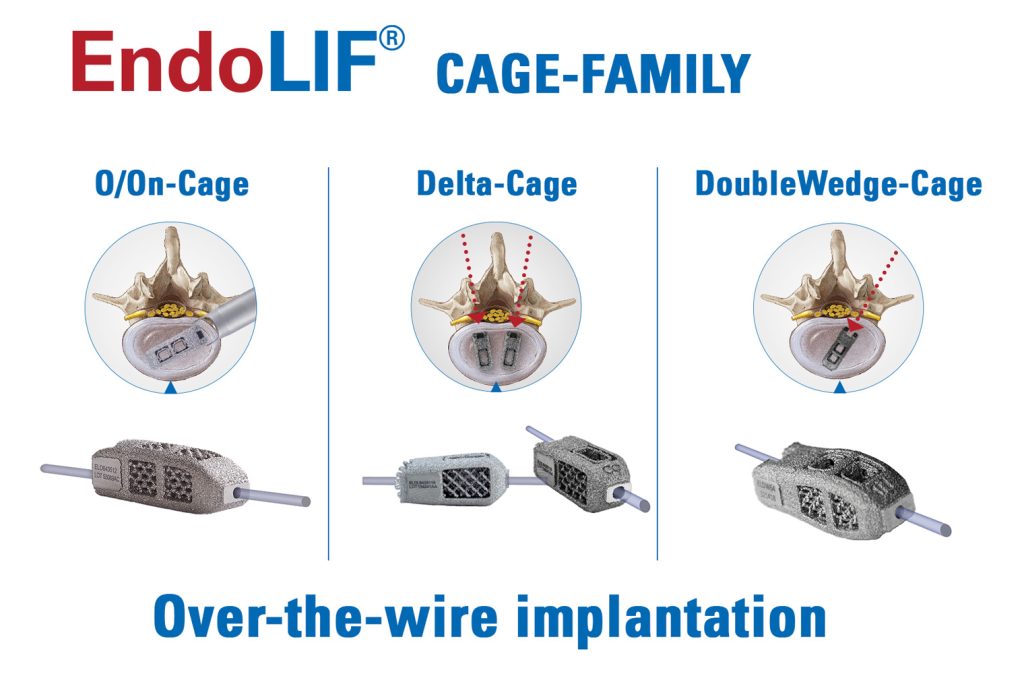
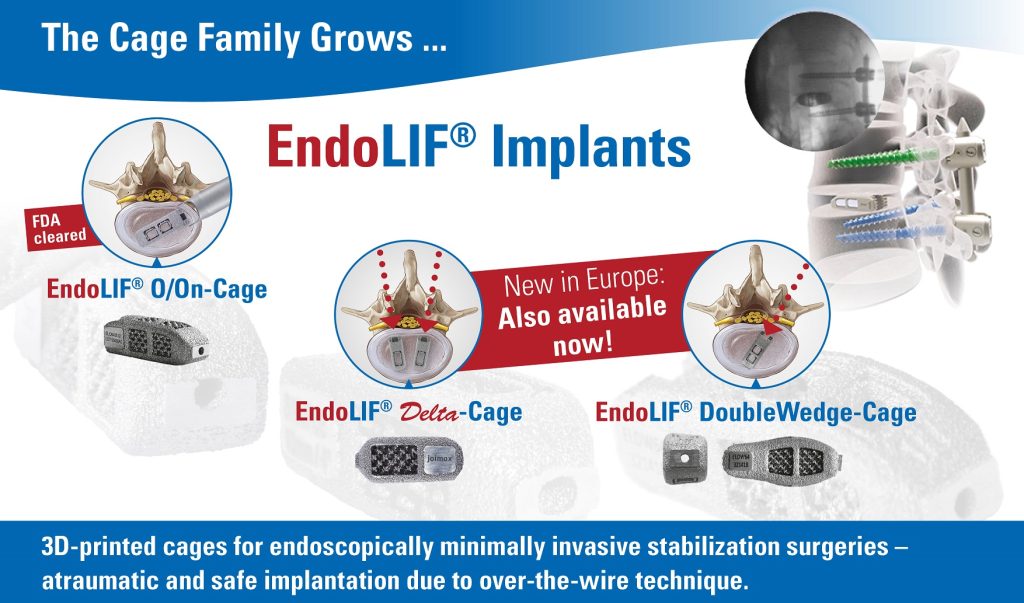
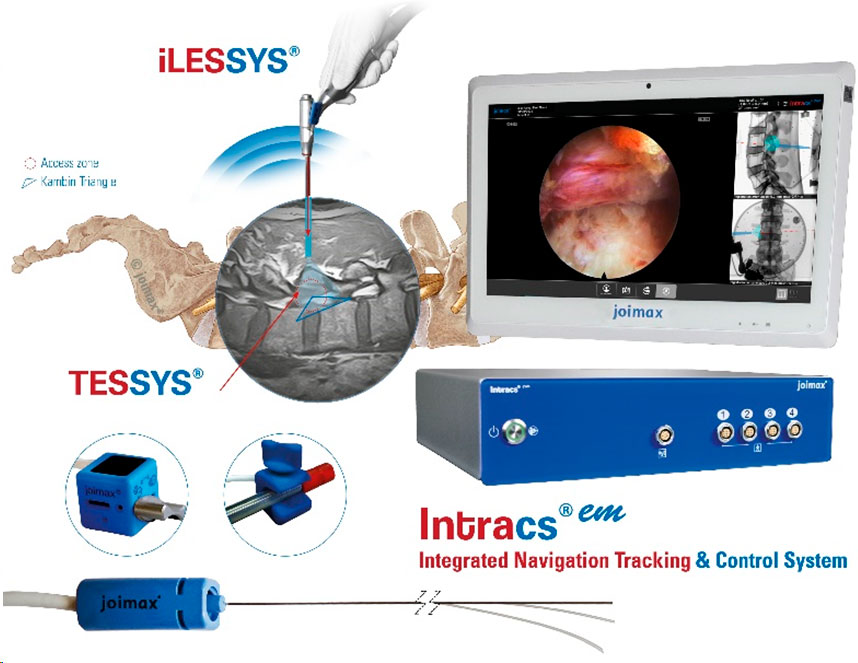
The Intracs®em electromagnetic navigation system is simple to set up, very user-friendly, and seamlessly integrates into the joimax® endoscopic tower. It allows for navigation during any endoscopic procedure performed with the joimax® endoscopic surgical systems, TESSYS® (transforaminal) and iLESSYS® (interlaminar). Beyond that, it can serve as a stand-alone device.
The system relies on electromagnetic tracking, affording simultaneous navigation of multiple instruments such as needles, guiding rods, reamers and endoscopes. It was developed by joimax® for easy planning of endoscopic approaches to the spine, as well as other minimally invasive procedures, like percutaneous fusions.
Various Intracs®em sensors guarantee the highest accuracy. Plus, for the patients’ benefit, the procedure can be carried out without further X-ray control – only starting X-rays are required. As a result, access, intervention time, and radiation exposure, are reduced to a minimum.
The system is CE-Marked. Currently, clinical trials and applications are running in Europe and Asia, where the product has already launched. In specific Asian countries, like Taiwan, the first systems have been sold and shipped.
Users are very impressed with the usability of the system. “I didn’t expect that navigation for both transforaminal and interlaminar procedures can be made so easy and that the set-up time can be so much streamlined,“ states Prof. Michael Kraus, spine specialist in Augsburg, Germany. “And for the thoracic approach, it is so precise and a tremendous help,” continues Dr. Erik Traupe, spine specialist in Munich, Germany.