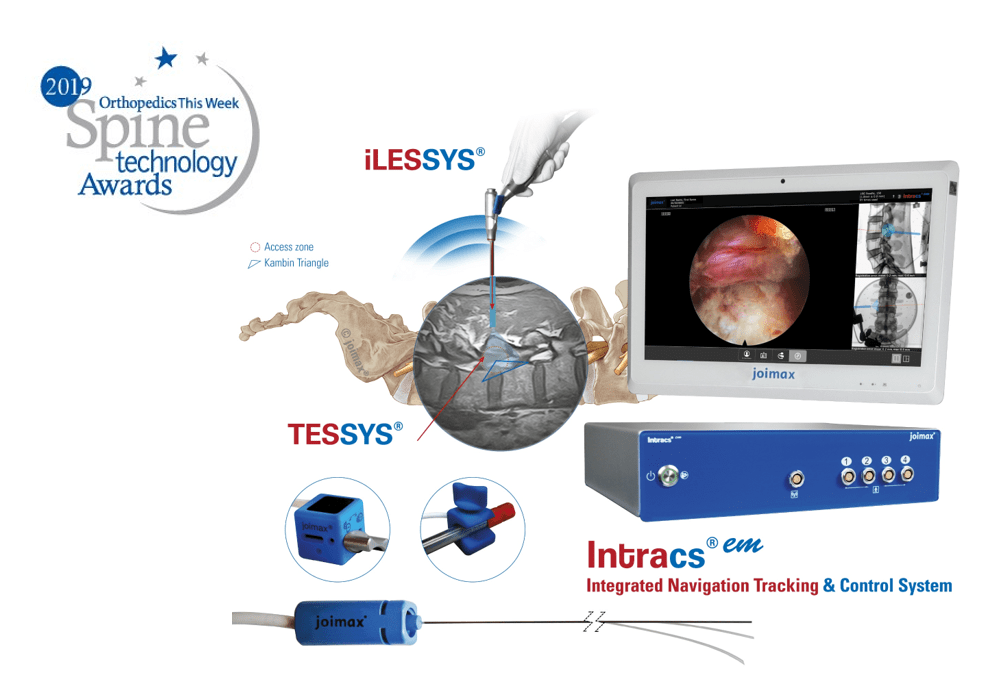
Irvine, CA – joimax®, a leading developer and marketer of complete systems for endoscopic minimally invasive spinal surgery, is pleased to announce they’ve won a 2019 Orthopedics this Week Spine Technology Award for their new Integrated Navigation Tracking & Control System, Intracs®em. joimax® will be recognized with the award during the annual North American Spine Society Meeting (NASS), taking place September 25-28 in Chicago.
joimax® is heavily involved in this year’s NASS program. On Wednesday, September 25, they will host three stations at the new, hands-on, full-day course, NASS/Neurospine Endoscopic Spinal Surgery Symposium and Cadaver Workshop, co-sponsored by KOMISS. Moreover, joimax® will present the latest technologies at their booth, No. 3416, including Intracs®em, the first electromagnetic system for endoscopic spine applications. Earlier this year, Intracs®em received its CE mark and is already very successful within Europe and Asia. It is pending FDA clearance; expected before year end.
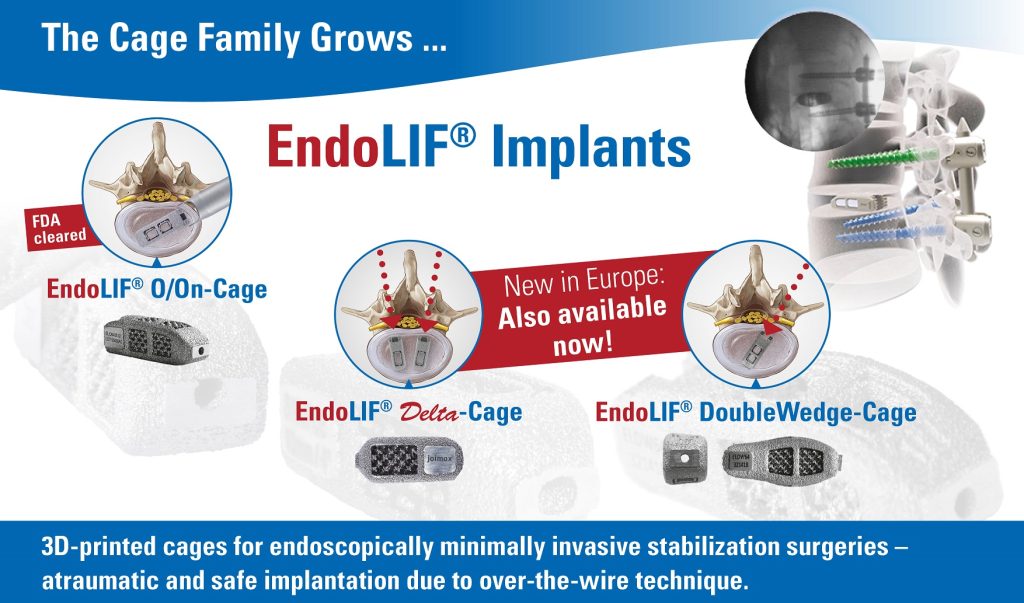
joimax® is also presenting ESPINEA®, Endoscopic Spine Academy, with its complete joimax® endoscopic education curriculum, which will play a crucial role in educating the next generation of endoscopic spine surgeons. Additional highlights in the booth will be the 4K Endoscopic tower and its optimal solution for endoscopic spinal surgery, as well as the latest 3D-printed EndoLIF® Cage family, consisting of the EndoLIF® O/On-Cage, DoubleWedge-Cage and Delta- Cage.
Wolfgang Ries, joimax® CEO and Founder states, “We are excited about the most recent developments within the US market. We are experiencing a great movement towards joimax® procedures, such as TESSYS® and iLESSYS®, from academics as well as private institutions. With our recently established ESPINEA® and the support from societies such as NASS, we believe we are at the tipping point where joimax® endoscopic spine surgery will become the gold standard of treatment in the US market.”